Vault Telehealth, Diagnostics & Clinical Trials
Supported COVID testing partnerships with more than 3,000 world-class customers to deliver care to individuals and larger populations during the pandemic. Launched and branded a new clinical research division, creating a distinct identity within the life sciences space while preserving brand consistency with Vault’s telehealth and diagnostic services.
Years
2021-2022
Client
Vault Health
Overview
Vault Health began in 2019 as a telehealth company focused on men’s health, offering diagnostics and treatment through virtual care. But when the COVID-19 pandemic hit, the company moved quickly to respond to the growing public health emergency. With a national network of medical providers, lab partners, and delivery infrastructure already in place, Vault was uniquely positioned to scale up testing fast. The team launched the first FDA-authorized at-home saliva COVID test, which allowed users to take the test under live supervision from a Vault clinician. This helped people across the country get back to work, school, sports, and other activities safely. Vault partnered with employers, schools, state and local governments, and major organizations, and played a key role in public health reporting. The company also made the first known detection of the Omicron variant in New York City.
At the same time, Vault was building out its life sciences division to tackle deeper problems in the clinical trial process. Many trials weren’t accessible or diverse enough, and that slowed down how quickly new treatments could reach the people who needed them. Vault used its existing systems to support decentralized trials that people could take part in from home, making the process more inclusive and efficient. My role during this time was to help tell Vault’s evolving story. I worked closely with clients and communities using our services, managed communications with the media, and helped keep the public informed when COVID cases were identified.
Key Timeline of Events
Clinical Logistics: COVID-19 Solutions
Healthcare screening, diagnostics and logistics management for Enterprise and Public Health.
Key Solutions & Services
Diverse testing options
Telehealth and/or on-site
Sample collection
Logistics management
Central lab capabilities
Data management
Customized programs
Fast & compliant reporting
Insurance billing
Key Industries Served
Enterprise
Public health
Edudation
Direct-to-consumer
10 Million+
Covid-19 tests sold
500K+
Vaccines administered
-
Individual and pooled PCR, rapid molecular, and rapid antigen
-
At home via Zoom, onsite with Vault staff, or self-managed by any school or organization
-
PCR: 24-48 hours
Rapid molecular: 30 minutes
Rapid antigen: 15 minutes
-
1,200+ highly trained, empathetic, and vetted clinical staff.
-
Flexible scheduling options and staffing solutions, with ability to execute quickly and customize to need.
-
Inventory management for collection kits and PPE; medical waste management; on-site technology management.
12 billion+ earned media impressions during the COVID pandemic
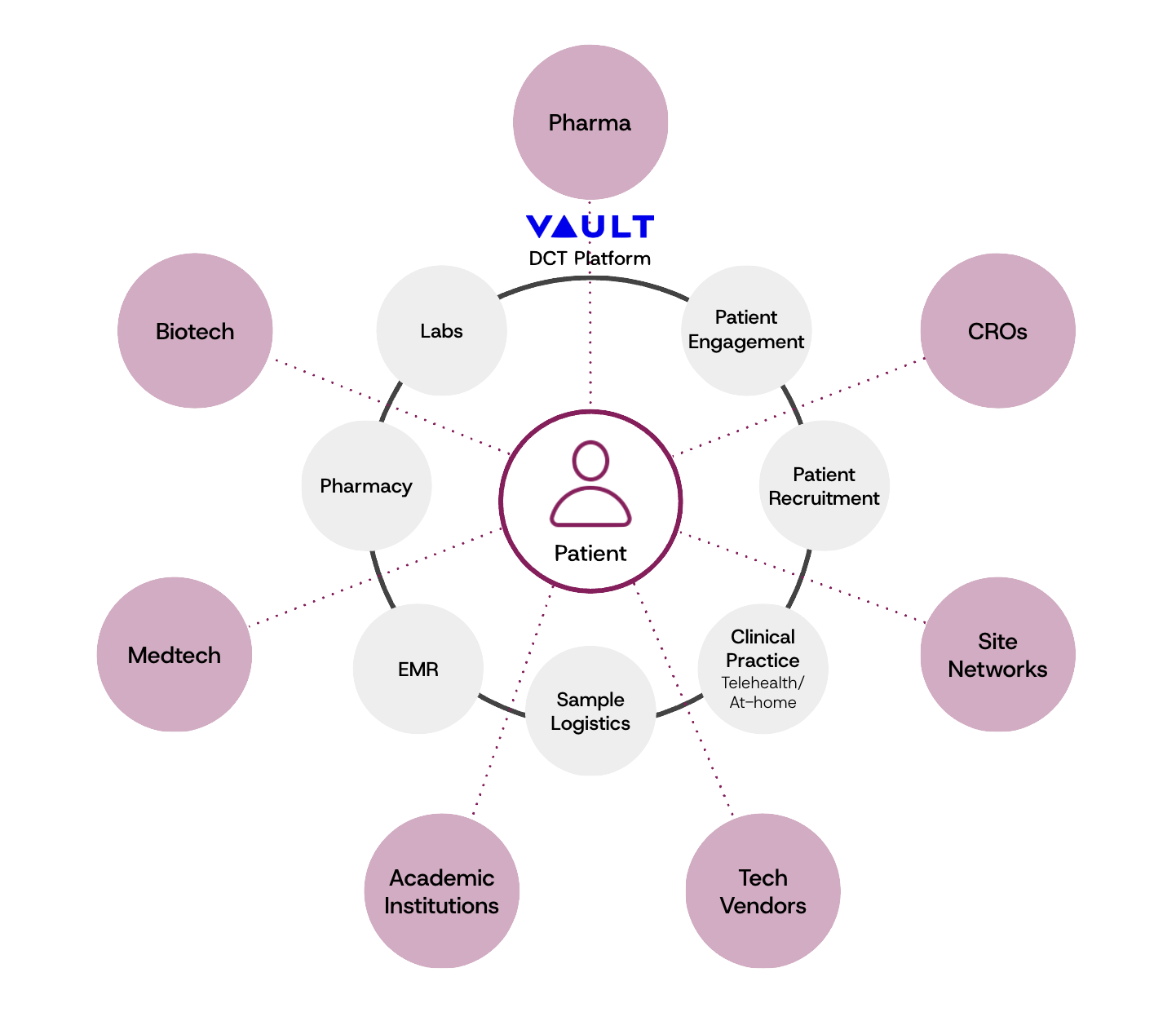
Clinical Research: Life Sciences Solutions
End-to-end decentralized and hybrid clinical trials management for Pharma, Biotech & Medtech.
Patient recruitment
Virtual study consent and enrollment
ECOA/EPRO
Patient engagement and retention
Logistics management
Central lab capabilities
Pharmacy capabilities
Data management
Post-marketing and RWE
Key Solutions & Services
Pharmaceutical
Biotech
Medtech
CROs
Academic institutions
Key Industries Served
Writing Samples
How Vault Powers DCTs That Promote Efficiency and Diversity
Innovation in the structure and design of clinical trials can help make them more efficient, relevant, and even diverse. These things are necessary for improving patient and physician experience while also creating opportunities for more meaningful outcomes.
Vault’s decentralized approach was intentionally designed to make it easier for patients to participate in clinical trials that are studying the effectiveness of new treatments. Our structure and flexibility helped us excel in speed, agility, and retention -- all of which contributed to more complete data in clinical research.
Why DCTs Are So Important for Patients in "Healthcare Deserts"
Millions of Americans live in rural communities (or “healthcare deserts”) where they don't have access to essential healthcare services. The makeup of rural populations across different U.S. regions is very intricate and directly influenced by government laws and policies over several decades. The rural healthcare crisis is growing. As these populations continue to age, the hospitals that serve these communities are facing more challenges that put them at risk of closure. Decentralized Clinical Trials help remove barriers to access and can ultimately provide better health outcomes to rural residents.
How RWD and RWE Can Improve Health Outcomes
Randomized clinical trials are the traditional method for observing patients as well as the safety and efficacy of interventions – but there are other tools at our disposal for making studies more efficient and effective. There is a growing interest around real world data and real world evidence given their ability to address a broader population with greater insights, more quickly. These tools can ultimately lead to better health outcomes and deliver impact at scale.
How Vault Helped Run a Convenient and Successful COVID-19 Immunity Clinical Trial
While Rutgers University handled the probiotic and sample testing, Vault led recruitment, logistical planning, and coordination for study subjects. At the start of the trial, we successfully recruited 54 people to participate over six weeks. The participants were then divided into three groups, receiving either a high-dose or standard dose of OL-1 probiotic supplements, or an inactive placebo.
To maximize convenience and flexibility, our virtual infrastructure enabled study subjects to participate in the trial from the safety and comfort of their own homes, taking supplements daily and providing biological samples through mail and via brief, COVID-safe home visits by clinical trial staff. There was no travel required, no cost to take part, and participants were compensated for their time. We also connected participants with researchers virtually online and kept journals of any symptoms they were experiencing during the trial.
Note: Vault Health has undergone several acquisitions for different parts of the business following the height of the COVID pandemic. Their Life Sciences platform was acquired by Science37 in 2023. Their workforce solutions side of the business was acquired by Sterling in early 2024, and Sterling was then acquired by First Advantage later that same year.